
Cheryl Koehn
Founder & President,
Arthritis Consumer Experts

Dr. Janet Pope
Rheumatologist, St. Joseph’s Health Care

Brad Hickling
Lives with Rheumatoid Arthritis
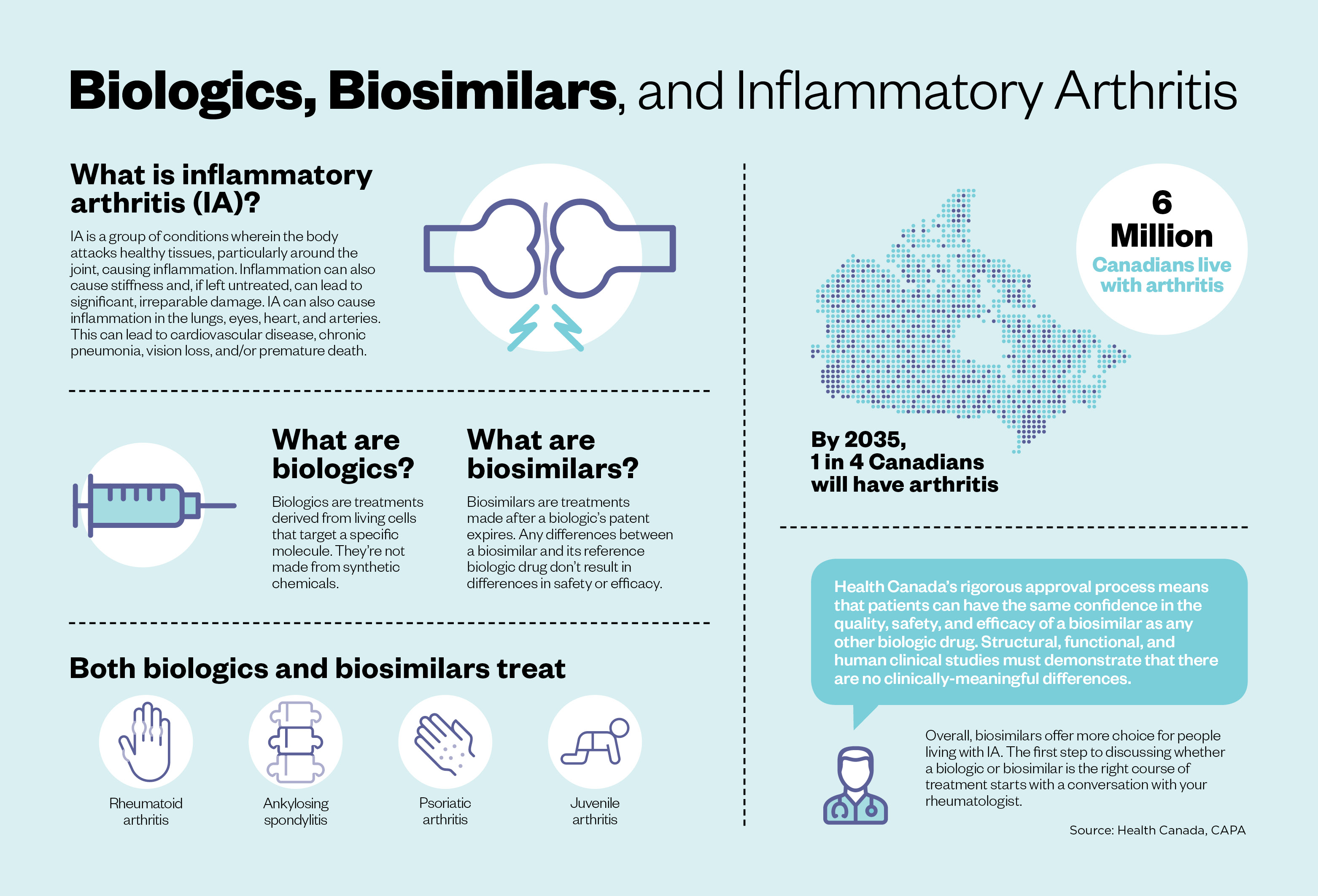
There are more than 300,000 Canadians living with rheumatoid arthritis, an autoimmune disease that causes joint pain and damage throughout the body. Without the right treatment, chronic pain and disability are the unfortunate consequences.
Cheryl Koehn knows chronic pain and disability too well. Once a high-performance athlete, she was diagnosed with rheumatoid arthritis in her late 20s. “I couldn’t walk or chew food, but I never thought the symptoms were arthritis,” she says. “I lived in denial and got depressed. I was desperate to make it go away.”
Twenty years ago, Koehn founded Arthritis Consumer Experts, Canada’s largest national arthritis patient-led organization, where patients and researchers work together to fast track science. The big break for arthritis treatment and improved quality of life for patients came with the development of powerful medications called biologics. “When I started biologics, I could interlace my fingers with my husband’s for the first time,” says Koehn. “This allowed me to go from about 40 percent of normal function to 75 percent. I could play tennis and I took up cycling.”
Biologics and biosimilars: what’s the difference?
As much as biologic medicines were a transformative treatment for those with arthritis and other diseases two decades ago, biosimilars have been similarly so.
Both originator biologics and biosimilar biologics are medicines developed from living cells that target a specific molecule. When the patent of an originator biologic expires, other manufacturers are allowed to make a biosimilar version of the medicine. There are no expected clinically-meaningful differences in efficacy and safety between a biosimilar and the originator. Biosimilars go through a rigorous review and testing process by Health Canada and other regulators around the world to ensure they’re safe and effective.
“Biosimilars deliver the same patient outcomes as originator biologic medicines. It’s amazing to see the scientific data that demonstrates the similarity between the two medications,” says Koehn. “One isn’t superior to the other, except when it comes to cost. That’s important as the savings generated by biosimilars may be reinvested into health care resources needed by Canadian patients.”
Biosimilars deliver the same patient outcomes as originator biologic medicines. It’s amazing to see the scientific data that demonstrates the similarity between the two medications.
The biosimilar transition
Biologic medicines are time-consuming and expensive to develop. This can make it difficult for the health care system to afford them, and limits patients’ access to biologics. Biosimilars can be up to 50 percent less expensive than the originator. This means that more patients will have access to these life-changing treatments, while not needing to stress about being burdened by high drug costs.
To date, two provinces — British Columbia and Alberta — have transitioned patients covered under public drug benefit programs to biosimilars. Many provinces, including Ontario and Quebec, are considering the same policies. Even many private health plans that many Canadians have through their employers are limiting the number of biologics they will cover or are implementing an annual cap or lifetime limit. Given that biologics can cost $20,000 to $35,000 a year, such coverage limits could present a financial burden for individuals.
Patient-approved
“Biosimilars have now been available for years and there are many studies and real-world patient data showing that the outcomes are the same when people are switched, including in Europe, and in parts of Canada,” says rheumatologist Dr. Janet Pope. “Switching to a biosimilar shouldn’t be something for patients to fear.”
Sixty-four-year-old Brad Hickling of Barrie, ON has struggled with inflammatory arthritis for much of his life. A few years ago, his rheumatologist asked if he would consider switching from his originator biologic to its biosimilar version. “I was nervous because I’d been almost pain-free for the seven years I was on the originator, but I trusted my rheumatologist and the information he provided,” says Hickling. “Transitioning to the biosimilar was seamless.”
This page was brought to you by a leading research-based pharmaceutical company.


